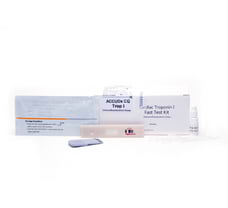
Cardiac Troponin I Kit (cTnl) 10 Tests, AccuDX CQ Troponin 1 Test Card
- Brand: Accurex Biomedical
- Catalog No.: AB16
- Quantity/Unit: 10 Tests/Box
- Usually Shipped in: 1-2 Weeks
Min Orderable Qty : 1 Pack
For lab/research use only, unless otherwise specified
ACCUDx CQ Cardiac Troponin I Kit (Immunofluorescence Assay) is intended for in vitro quantitative determination of Cardiac Troponin I (cTni) in serum, plasma or whole blood. This test is used as an aid in the diagnosis of myocardial injury such as Acute Myocardial Infarction (AMI), Unstable Angina, Acute Myocarditis and Acute Coronary Syndrome (ACS). Troponin, a molecular complex that is bound to the thin filament (actin) of striated muscle fibers, acts with intracellular calcium to control the interaction of the thin filament with the thick filament (myosin), thus regulating muscle contraction. Troponin consists of three regulatory proteins: T, which connects the troponin complex and tropomyosin (another cardiac muscle regulatory protein); I, which prevents muscle contraction in the absence of calcium; C, which binds calcium. Cardiac Troponin I (MW 22.5 kDa) and the two skeletal muscle isoforms of Troponin I have considerable amino acid sequence homology, but cTni contains an additional N-terminal sequence and is highly specific for myocardium. Clinical studies have demonstrated the release of cTnl into the blood stream within hours following acute myocardial infarction (AM) or ischemic damage. Elevated levels of cTnl are detectable in blood within 4 to 6 hours after the onset of chest pain, reaching peak concentrations in approximately 8 to 28 hours, and remain elevated for 3 to 10 days following AM. Due to the high myocardial specificity and the long duration of elevation, Tni has become an important marker in the diagnosis and evaluation of patients suspected of having an AMI. The current quidelines cf The Joint European Society of Cardiclogy/Amarican College of Cardiology Committee support the use DicTni as a preferred marker of myocardial injury. Several major studies have shown that cTni is also a predictor of cardiac risk in patients with unstable angina. The American College of Cardiology and the American Heart Association's current guidelines recommend using troponin results when making treatment decisions regarding unstable angina and non-ST segment elevation MI (NSTEMI). TEST RESULTS: ACCUDx CQ Analyzer can scan the test card automatically and display the result on the screen. For additional information, please refer to the user manual of ACCUDx CQ Analyzer